Impact of Unexplored Data Sources on the Historical Distribution of Three Vector Tick Species in Illinois
Beth Gilliam, Peg Gronemeyer, Sulagna Chakraborty, Fikriyah Winata, Lee Ann Lyons, Catherine Miller-Hunt, Holly C Tuten, Samantha Debosik, Debbie Freeman, Marilyn O’hara-Ruiz ... Show more
Journal of Medical Entomology, Volume 57, Issue 3, May 2020, Pages 872–883, https://doi.org/10.1093/jme/tjz235
Published:
13 December 2019
Abstract
We updated the Illinois historical (1905–December 2017) distribution and status (not reported, reported or established) maps for Amblyomma americanum (L.) (Acari: Ixodidae), Dermacentor variabilis (Say) (Acari: Ixodidae), and Ixodes scapularis (Say) (Acari: Ixodidae) by compiling publicly available, previously unexplored or newly identified published and unpublished data (untapped data). Primary data sources offered specific tick-level information, followed by secondary and tertiary data sources. For A. americanum, D. variabilis, and I. scapularis, primary data contributed to 90% (4,045/4,482), 80% (2,124/2,640), and 32% (3,490/10,898) tick records vs 10%, 20%, and 68%, respectively from secondary data; primary data updated status in 95% (62/65), 94% (51/54) and in 90% (9/10) of the updated counties for each of these tick species; by 1985 there were tick records in 6%, 68%, and 0% of the counties, compared to 20%, 72%, and 58% by 2004, and 77%, 96%, and 75% of the counties by 2017, respectively for A. americanum, D. variabilis, and I. scapularis. We document the loss of tick records due to unidentified, not cataloged tick collections, unidentified ticks in tick collections, unpublished data or manuscripts without specific county location, and tick-level information, to determine distribution and status. In light of the increase in tick-borne illnesses, updates in historical distributions and status maps help researchers and health officials to identify risk areas for a tick encounter and suggest targeted areas for public outreach and surveillance efforts for ticks and tick-borne diseases. There is a need for a systematic, national vector surveillance program to support research and public health responses to tick expansions and tick-borne diseases.
tick, distribution, establishment, surveillance, Illinois
Subject Editor: Maria Diuk-Wasser
Research and published efforts have not identified or used all the historical information on vector ticks (Acari: Ixodidae) in Illinois to update temporal and spatial distribution and status maps of the three dominant tick vectors of public health importance in Illinois: the lone star tick, Amblyomma americanum; the American dog tick, Dermacentor variabilis; and the blacklegged or deer tick, Ixodes scapularis (CDC 2018).
Across the United States and Illinois, the geographic distributions of these three species have expanded in recent decades (Bouseman et al. 1990, Dennis et al. 1998, Rydzewski et al. 2012, Eisen et al. 2016, Nieto et al. 2018). Based on climate models, continued range expansion is predicted for D. variabilis (Minigan et al. 2018), which is assumed to be widespread in Illinois (CDC 2019b). Amblyomma americanum has expanded its range northward into the upper Midwest, including Illinois (Monzón et al. 2016). Ixodes scapularis has spread south into Illinois from Wisconsin since the late 1980s, and likely from the east since the late 1990s (Eisen et al. 2016).
Human diseases caused by tick-borne pathogens have increased globally in recent decades (De la Fuente et al. 2008, Dantas-Torres et al. 2012). Lyme disease is the most common tick-borne illness in Illinois and the rest of the United States (Herrmann et al. 2014). Other frequently diagnosed tick-borne illnesses in Illinois residents include Spotted Fever Rickettsioses, Ehrlichiosis, Anaplasmosis, and Tularemia (IDPH 2018a).
Tick hosts such as white-tailed deer (Odocoileus virginianus) (Cortinas et al. 2006), small mammals, and birds (Hamer et al. 2012) are responsible for the movement of ticks (Sotala et al. 1973, Anderson et al. 1984, Lantos et al. 2017). Host movements vary with year and season, affecting tick expansion and distribution in the landscape (Gill et al. 2001, Allan et al. 2010).
Tick survival depends on habitat characteristics and weather (Kitron et al. 1991, Guerra et al. 2002, Schulze et al. 2009, Gilliam et al. 2018). Ticks rely on temperature to regulate rates of development and on humidity to regulate survival. A tick’s ability to capture water from the atmosphere is modulated by remaining in vegetation closer to the ground to conquer periods of low relative humidity (Estrada-Peña and de la Fuente 2014). Habitat suitability models predict areas suitable for the establishment of I. scapularis across Illinois (Brownstein et al. 2003, Cortinas et al. 2006, Hahn et al. 2016) and should take into account weather, habitat and landscape characteristics.
There are spatial distribution maps of ticks with county-level information using active and passive surveillance published for Oklahoma (Barrett et al. 2015, Mitcham et al. 2017), Nebraska (Cortinas et al. 2014), and Iowa (Oliver et al. 2017). National spatial distribution maps, for I. scapularis (Dennis et al. 1998, James et al. 2015, Eisen et al. 2016, Nieto et al. 2018), A. americanum (Springer et al. 2014), and D. variabilis (James et al. 2015, Nieto et al. 2018) highlighted the status (established, reported, or not reported) for counties with available records. Existing distribution maps of tick vectors use heterogeneous data sources such as peer-reviewed literature, passive and active surveillance, entomological collections, and databases available for public access. We will focus on evaluating the impact of previously unexplored newly identified published and unpublished data (untapped data) on the historical distribution of three dominant tick vectors of public health importance in Illinois, A. americanum, D. variabilis and I. scapularis. Our objectives are to 1) compare published publicly available statewide distribution data from national distribution maps of A. americanum, D. variabilis I. scapularis, with previously unexplored, newly identified published and unpublished (untapped data) data sources; 2) update county-level distribution maps for the three main vector ticks in Illinois; 3) identify counties in Illinois in need of additional tick surveillance; and 4) illustrate the impact of untapped data on the historical tick distribution maps of these ticks in Illinois.
Materials and Methods
We collected, extracted, and compiled tick collection data for the three main tick vector species in all counties in Illinois: A. americanum, D. variabilis, and I. scapularis (previously I. dammini) expanding from 1905 through 31 December 2017. We collected data from peer-reviewed manuscripts, researchers, and tick or entomological collections that were either publicly available; or unreported to public collection databases. Entomologists and tick researchers identified old specimens from their collections and accessioned the data for this work.
For the identification and inclusion of data from manuscripts we conducted literature searches using Scopus (Elsevier.com), PubMed and Web of Science (Thomas Reuters, NY) with the following keywords: (tick* AND ‘Amblyomma americanum’ OR ‘Lone star tick’ OR ‘Dermacentor variabilis’ OR ‘American dog tick’ OR ‘wood tick’ OR ‘Ixodes scapularis’ OR ‘deer tick’ OR ‘blacklegged tick’) AND ‘Illinois’. We screened for and removed duplicate manuscripts and tick records. We selected manuscripts based on the inclusion criteria (detailed below), downloaded, read, and assessed the complete text of the manuscripts for eligibility. We included all those manuscripts with specific Illinois county-tick data that provided specific tick numbers, or that allowed for the determination of a minimum number of ticks by species in that county within a year or a range of years.
We complemented this effort with a snowball technique and examined the referenced materials of the selected manuscripts as in Springer et al. (2014) to maximize the records evaluated; this leads to the inclusion of additional manuscripts.
The inclusion criteria comprised peer-reviewed manuscripts published before December 2017 who provided specific information on one of the three focal tick species, including county-specific tick data and year or range of years of tick collection. If more than one paper by the same author(s) utilized the same data, we selected the earliest manuscript as our data source. If we identified a dataset created and cited by one researcher as utilized in a manuscript written by a different researcher, we selected the original publication of the data as our data source.
Thirty-one papers met our criteria and were used to map tick occurrences (Figs. 1 and 2) and to summarize the available information obtained from the data sources (Tables 1–3). However, we included manuscripts with national tick distribution data and maps published before 1 August 2018. The information from these national distribution manuscripts (Dennis et al. 1998, Springer et al. 2014, James et al. 2015, Eisen et al. 2016, Nieto et al. 2018) contained information for all the counties in the state of Illinois; we used the Illinois data from these national distribution manuscripts to develop our baseline or ‘original’ statewide distribution and status for A. americanum, D. variabilis, and I. scapularis. We extracted data from the remaining manuscripts to build the dataset used to compare with the baseline information, and updated the distribution and status of A. americanum, D. variabilis, and I. scapularis in Illinois.
Table 1.
Primary data sources providing ‘raw’, non-aggregated specific tick-level data with collection location for Amblyomma americanum, Dermacentor variabilis and Ixodes scapularis
Fig. 1.
Open in new tab Download slide
First reports of three common vector ticks in Illinois from 1905 to 2017.
Fig. 2.
Open in new tab Download slide
Snapshot of vector tick distribution. Colored counties in red, orange, and yellow indicate updated status as of 31 December 2017. Original species status determined by five summary papers is in gray or white. We changed the grayscale to red, orange, or yellow depending on specific status update. *UTD status= updated status from this work.
We expanded our data collection by contacting lead researchers from the 26 peer-reviewed publications identified. We excluded researchers with ticks from avian hosts to minimize tick data from neighboring counties or states, and the five national tick distribution papers informing our baseline. We included researchers whose manuscripts suggested that there was tick-level data for counties where our baseline information was not established.
We requested access to these researchers’ tick-level collection data for all counties available, if they were in a position to share data and if the data was available or could be made available at the time of this work. We expanded data gathering efforts for all counties in Illinois by using the United States National Tick Collection (USNTC) and the ‘TickReport’ a Tick-borne Diseases Passive Surveillance (https://www.tickreport.com/) program. We also used the state of Illinois Department of Public Health (IDPH) data, which collects data across all the counties in Illinois.
Based on personal communications during past and current collaborations, we used our existing knowledge and contacted known sources of tick-level data and collections that were not publically available at the time of this work. This included contacting local officials in Kendall County Health Department (KCHD) and the Forest Preserve District of DuPage County (FPDDC), both known to have conducted independent tick dragging and data collection efforts prior to 2017; and, we and requested a special effort to identify, accession, update and share their data with us. Similarly, we contacted the Illinois Natural History Survey (INHS)—Insect Collection and requested their effort to identify and catalog tick specimens known (based on personal communications) with a collection date in Illinois before 31 December 2017, not yet identified. Furthermore, we identified the ticks from an existing historical collection effort belonging to one of the corresponding authors in this study and included that information in this work.
We separated data sources into primary, secondary, and tertiary categories based on the level of detail available from each source. We developed and followed decision rules to classify all the data sources into one of these three categories of data. Primary data exist as raw data at the individual tick-level originating from researchers and tick collections (not extracted from the published literature). Primary data required no interpretation to estimate the number of ticks and a tick status. They provided specific species, location for each tick collected, number of ticks, year and generally included life stage, sex, collection method, and host (Table 1).
We extracted secondary data from the text, figures, and maps of peer-reviewed manuscripts. These sources provided tick species, aggregated total number of ticks, county locations, approximate date of collection, or a range of 10 or fewer year(s) (Table 2), but we could not consistently determine the life stage for a single tick. Tertiary data sources included data from publications with established or reported species status by county but sometimes lacked specific year of tick collection or tick numbers (Table 3). If the data source did not provide the collection date, we used the year of publication as the collection date. Some tertiary data sources only provided enough information to determine the county of tick collection or to estimate a minimum number of ticks per county. For example, if the manuscript reported ‘…adult and nymph I. scapularis were removed from 49 infested deer…’ we assumed a minimum of 49 I. scapularis, although we did not have the exact number of adults or nymphs; we interpreted the words ‘infested deer’ as at least one tick per deer. If the data source did not provide specific tick information to help us characterize a tick species as established in a county (based on Dennis et al. 1998, and Eisen et al. 2016), but gave at least county and species information, we classified the county as reported.
Table 2.
Summary of secondary data sources providing aaggregated numbers of ticks (Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis) for a county at an approximate date of collection or range of collection years
Table 3.
Aggregated data provided by tertiary data sources
We established the following guidelines to maintain consistency while extracting secondary or tertiary data and converting them to discreet dates or locations. We chose the last year in a range of years reported as the actual ‘year of the report or establishment’. We considered a tick species established if the aggregate number of ticks provided information for a range of years in a county but, we required the minimum number of ticks to be six times the total number of collection years in the year range. For example, for ticks (of the same species found in the same location) collected from 2003 to 2006, at least 24 ticks (four collection years multiplied by six) indicated the species was established. We screened the data for duplicates by comparing records for identical collector names, curators, collection dates, species, and county. We removed duplicate records from the dataset if a single person’s records were found in two different data sources and removed records with the least amount of detail. If the data were very similar, but it was unclear that the tick collector was the same, we assumed that they were not duplicates and kept all records. Some data sources provided only a region, e.g., ‘Chicagoland’, instead of a county location. Without the number of ticks or county information, we could not determine or estimate the ticks found in a county and, therefore we did not include these sources in this work. These data sources did not pass out selection criteria to enter this study.
To depict spatial and temporal first reports and vector distribution status, we used ArcGIS 10.6 (ESRI, Redlands, CA) to map the first year of observation, or ‘first report’ (Fig. 1), and status (NR, R, or E) of A. americanum, D. variabilis, and I. scapularis. We followed the criteria used by Dennis et al. (1998) and Eisen et al. (2016), and if we collected two or more life stages or six or more individual ticks in a given county within the same year, we considered the tick species as established (E). If we found at least one tick species, we classified it as reported (R); if we did not detect tick records in a county, we recorded it as not reported (NR). Once a tick species was ‘reported’ in a county, it remained ‘reported’ unless new information became available, indicating a change to ‘established’. Once a tick species was ‘established’, the status did not change regardless of new data.
We summarized the data by species, year collected, county, and life stage (when available) to determine the occurrence and species status (E, R, or NR). We defined and mapped our original baseline status (R or E) of a tick species using the information from all the national distribution manuscripts and represented this baseline knowledge in grayscale (Fig. 2). We used our baseline or ‘original’ distribution data and compared it with the tick distribution information gained from the primary, secondary, and tertiary data. We symbolized the updated status by county in red, orange, and yellow. The updated status red indicated a change from NR to E, in orange for NR to R, and in yellow for R to E (Fig. 2). Counties not updated from this work remained in grayscale.
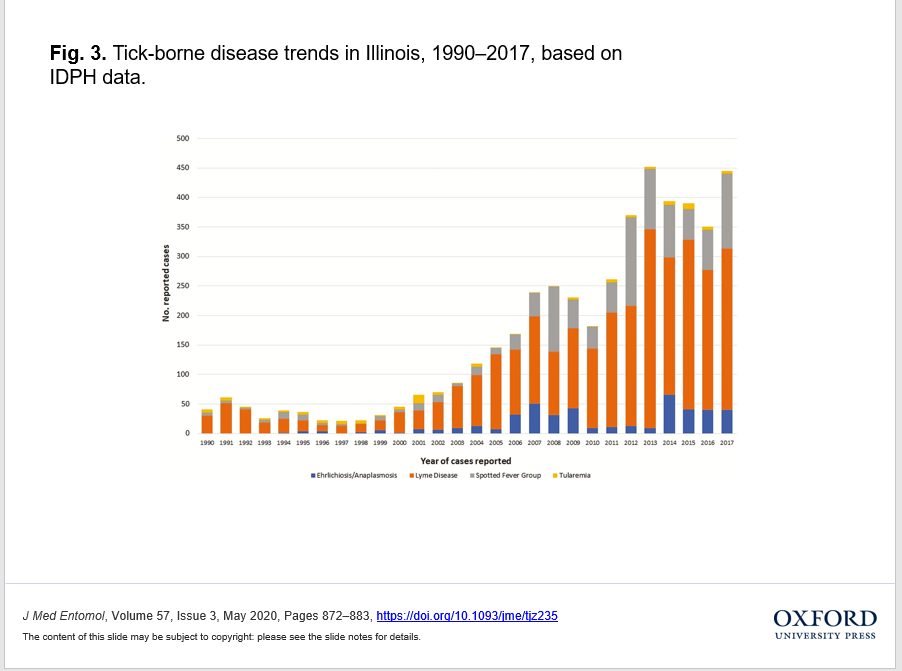
In order to create a temporal visualization of tick-borne disease trends in Illinois from 1990 to 2017 (Fig. 3), we used data from the CDC’s National Notifiable Infectious Diseases Surveillance System (CDC 2018), from the Illinois Department of Public Health (IDPH 2018a), and the tick-borne disease data from the CDC Morbidity and Mortality Weekly Reports (Adams et al. 2016, 2017). From all these sources, we extracted data on the most common five tick-borne diseases (Lyme disease, Ehrlichiosis, Anaplasmosis, Tularemia, and Spotted Fever Group Rickettsioses (SFGRs) associated with pathogens and transmitted by A. americanum, D. variabilis, and I. scapularis in Illinois.
Fig. 3.
Open in new tab Download slide
Tick-borne disease trends in Illinois, 1990–2017, based on IDPH data.
Results
We updated the year range of ‘first report’ (Fig. 1) and provided the distribution maps and the status no report (NR), reported (R) or established (E) at the county level (Fig. 2) for the three main vector tick species in Illinois A. americanum, D. variabilis, and I. scapularis.
We identified 18,020 tick records (Supp Table S1 [online only]) collected from 39 data sources, including 30 published references from 1905 to 2017 and one national distribution manuscript published in July 2018. We found at least one tick record for one of the targeted tick species in 101 of the 102 counties in Illinois. Stark County was the only county without tick reports.
The distribution of D. variabilis was documented in 70 counties in Illinois from first reports before 1985 (Fig. 1) in contrast to reports in seven counties for A. americanum and no reports for I. scapularis. Fourteen years later (by the end of 1999), there were no additional counties with reports for D. variabilis although, there were first reports in 10 counties for A. americanum and 52 counties for I. scapularis. By the end of 2009, there were reports in 67, 86, and 69 counties for A. americanum, D. variabilis and I. scapularis, respectively. By the end of December 2017, there were tick reports in 79, 98, and 77 counties for A. americanum, D. variabilis, and I. scapularis, respectively. Based on primary, secondary and tertiary information we identified the total number of counties with NR, R or E status as follows: for A. americanum (NR = 23, R = 50, E = 29); for D. variabilis (NR = 4, R = 65, E = 33) and for I. scapularis (NR = 25, R = 37, E = 40) (Fig. 2, Supp Table S1 [online only]).
By using previously unexplored, newly identified published and unpublished data (untapped data sources), we updated the status in 65, 54, and 10 counties for A. americanum, D. variabilis, and I. scapularis respectively. The number of counties with status updates for A. americanum was 40 from (NR to R), 19 from (NR to E) and six from (R to E); for D. variabilis they were 33 from (NR to R), nine from (NR to E) and 12 from (R to E); and for I. scapularis they were six from (NR to R), two from (NR to E) and two from (R to E). We represented the original species status by county in grayscale and the updates in colors red, orange, and yellow (Fig. 2).
Our search for records from published manuscripts followed the basic structure and rules of a systematic review. The initial searches yield n = 32 manuscripts in Pubmed, n = 168 in Scopus, and n = 69 in Web of Science; for a total of 269 results. After screening and removing duplicate records, 220 manuscripts remained, and only 22 met our inclusion criteria, but only 17 of those were eligible and included specific Illinois county-tick data with especific tick numbers or information that allowed for the determination of a minimum number of ticks by species in that county. After examining the referenced materials of these 17 manuscripts, we identified 13 more manuscripts published before 31 December 2017, that met our inclusion criteria. However, we included all national tick distribution maps published before 1 August 2018, in determining the original status and distribution maps for A. americanum, D. variabilis, and I.scapularis leading to the addition of one more manuscript. The total number of manuscripts used as data sources was 31.
We contacted researchers from the 26 peer-review publications (excluding the five national tick distribution papers) whose manuscript(s) suggested that there was tick-level data for counties with baseline reports different than established, we excluded researchers with tick information from avian hosts. Three researchers provided the tick-level data used in their manuscripts and included data omitted from their published work; one researcher could not recover old data but gave us data from more recent, but unpublished efforts and two lead researchers are now deceased.
Prior knowledge of tick-level data (from personal communications with past and present collaborators) contributed to 2,406 records. These records were in tick collections that, at the time of this work, had not been reported to public collection databases. Few records were not cataloged, or their ticks identified, but, were identified and accessioned in response to our request. These data sources did not always report the number of ticks newly identified or accessioned in response to our request included within the data given to us.
Data sources contributing enough information to determine tick species, location of county, year of collection, and status (R, NR, or E) yielded 11 primary data sources, 15 secondary data sources, and 13 tertiary data sources (Tables 1–3). Primary sources accounted for 9,659 individual tick records in 100 counties: 4,045 A. americanum in 77 counties, 2,124 D. variabilis in 94 counties, and 3,490 I. scapularis in 46 counties (Table 4). Primary sources that received voluntary tick submissions provided information for the three tick species of interest and covered more counties than data sources using methods for tick collections that included dragging or fieldwork with vertebrates. Four of the primary data sources focused on a single county, two provided information on three and five counties, and five sources provided tick reports for 35 or more counties (Table 1).
Table 4.
Cumulative number of ticks (by species) and counties identified from primary, secondary and tertiary data sources
Primary data sources reported all three life stages (adult, larva, and nymph) except for KCHD, who only reported adults and Kitron et al. 1991, who only reported larvae. We found the A. americanum by the end of 2017 established in 29 counties, 12 of those based on life stage information; Ixodes scapularis established in 40 counties, (six counties, based on life stage data). We found few D. variabilis larva or nymphs, so although this species is established in 33 counties, we only determined the established status, based on the life stage for two counties.
We attributed most of the updates to the distribution and status for all three species to previously untapped primary data sources. Of the 11 primary sources, those that housed tick collections or received voluntary tick submissions for passive surveillance efforts, identification, and or pathogen testing (N.E. Mateus-Pinilla, INHS [2018] entomological collection, USNTC [2019], IDPH, and TickReport [2019]) provided 4,700 tick records for ≥ 35 counties. Individual researchers sharing their data (M.E. Gilliam, J. Rydzewski, U. Kitron, and J. Nelson) accounted for 4,959 tick records covering one to five counties (Table 1).
Primary and secondary data sources provided the specific number of ticks and county location although, primary data sources offered more details for a tick record. In comparison, primary data for A. americanum, D. variabilis, and I. scapularis contributed to 90% (4,045/4,482), 80% (2,124/2,640), and 32% (3,490/10,898) of all tick records vs 10%, 20%, and 68% respectively from secondary data. Secondary sources accounted for 8,361 individual tick records across our three tick species of interest, 89% (n = 7,408) were I. scapularis (Table 4) and focused on one or two counties, except for Cortinas and Kitron (2006) and Nieto et al. (2018), who collected from 17 to 46 counties, respectively. Five secondary sources identified 437 A. americanum in 14 counties, five reported 516 D. variabilis in 35 counties, and 11 reported 7,408 I. scapularis in 35 counties. Secondary sources offered more information about tick hosts compared to primary or tertiary data sources (Table 2).
Tertiary data sources provided an aggregate number of ticks, or an opportunity to estimate a minimum number of tick records from the text, tables, or figures, and provided categorical information designating a county as established or reported with collection-year or range of collection years. These tertiary sources contributed to the information in 11, 33, and 65 counties for A. americanum, D. variabilis, and I. scapularis, respectively (Table 4).
Of the 13 tertiary data sources, four were national distribution maps used to develop our original baseline status and county-level distribution maps. Three of the national distribution maps and two other tertiary sources, did not provide specific tick numbers. For eight of the tertiary data sources we were able to estimate a minimum number of ticks for a species in a county after reading the text and evaluating figures and tables. For one data source, we used the year of publication to indicate the relative year of tick collection based on the information in the text. Twelve tertiary data sources provided aggregate tick data between collection years and no specific year of the collection (Table 3). We present the estimated minimum number of ticks by species from tertiary sources (Tables 3 and 4).
Counties in need of surveillance by target tick species include those without tick reports 23, 4, and 25 for A. americanum, D. variabilis, and I. scapularis, respectively (Fig. 2). We found 62 counties with a tick report for each of the three tick species; However, 29 counties have an NR status for one of the three ticks species; 10 counties have an NR status for at least two tick species (Alexander, Christian, Clay, Douglas, Effingham, Ford, Massac, and White Counties have an NR status for A. americanum and I. scapularis; and Hamilton and Jefferson have an NR status for both D. variabilis, and I. scapularis). To date, Stark county is the only county without a single tick report for any of the three tick species (Supp Table S1 [online only], Supp Fig. S1 [online only]).
Discussion
This study used 18,020 tick records (Supp Table S1 [online only]), covering over 100 yr of data. We used a data inventory effort from data sources derived from the literature, entomological collections, and researchers willing and able to finalize old specimen identifications and to share their data with this project. By consolidating information from several data resources, we illustrated temporal changes in the first reports and spatial distribution maps of the three main vector ticks in Illinois, A. americanum, D. variabilis, and I. scapularis, as of December 2017. While the data used for this manuscript comes from the use of various strategies’ in the collection of ticks across time and space, our work provides a critical update to the distribution, status, and knowledge of the first reports of these three tick species in Illinois.
Temporal changes in the timing of first reports observed from our results suggest under-reporting before 1985, an increase in tick occurrence and numbers over time, geographical expansion of these vectors, or new establishment for A. americanum and I. scapularis after 1985 (Fig. 1).
Even though we documented the wide distribution of these tick species in Illinois, we could assume that counties without a report for A. americanum, D. variabilis, or I. scapularis (23, 4, and 25, respectively) (Fig. 2) surrounded by counties with ticks (Supp Table S1 [online only], Supp Fig. S1 [online only]) are likely to have ticks. These gaps may relate to lack of surveillance and tick data from these counties, and there may be something unique about these counties influencing the occurrence of these ticks. Thus, the need for surveillance and research in these areas.
Dermacentor variabilis can be a vector for Rickettsia rickettsii (the agent of Rocky Mountain spotted fever) and Francisella tularensis (the agent of Tularemia) (CDC 2019c). Eight published manuscripts provided data on D. variabilis in Illinois (Tables 2 and 3). Cases of these illnesses are increasing in Illinois; however, the extent of human exposure to these ticks requires further assessment (Herrmann et al. 2014). The status updates from this manuscript facilitate directing future studies, disease, and tick surveillance efforts and assessments of risk of tick exposure.
Similarly to Wisconsin, Illinois saw a southwestern range expansion of I. scapularis, and an increase in density of I. scapularis on white-tailed deer during the late 1980s (Bouseman et al. 1990, French et al. 1992). Ixodes scapularis was first found in northwestern Illinois in the late 1980s. Therefore, it is assumed that the density of I. scapularis could be higher in the northern half of the state due to its proximity to areas where the tick was introduced (Cortinas et al. 2006). Our data support that the majority I. scapularis records came from the northern half of the state (Fig. 1); in agreement with a map from the CDC (2019b) supporting the wide distribution of I. scapularis in the northern regions of Illinois. Our observed increased number of records for I. scapularis during the last two decades may be due to increased passive surveillance in response to increases in public health concerns related to Lyme and other tick-borne diseases in Illinois and the United States.
We found A. americanum throughout the state of Illinois, although most of the reports were after 1985. The increased reports and updated distribution status of this tick species in Illinois have significant implications for public health. Amblyomma americanum is associated with the emerging vector-borne diseases STARI–Southern Tick Associated Rash Illness, Tularemia, and Ehrlichiosis which may have contributed to increased public interest, awareness and concerns leading to increased tick submissions during the last 20 yr (Mixson et al. 2006, Goddard et al. 2009, Tokarz et al. 2014). We present the reported cases of some of these human tick-borne diseases in Illinois in Fig. 3. We assume that the impact of these diseases on public health contributes to a surge in tick research that likewise influences tick submissions, identification efforts, and reporting. Besides, we assume that the aggressive, generalist feeding behavior observed at all life stages of A. americanum (Childs and Paddock 2003) and the growing range expansion of this tick in Illinois, will facilitate public awareness and submission of A. americanum specimens. Continuing to intensify the impact of this tick on human health in Illinois are the first reports of Heartland virus (IDPH 2018b) in 2018 in Illinois, and reports of Alpha-Gal Syndrome, an allergic reaction to the consumption of red meat following the tick bite from an A. americanum (Fischer and Hilger 2017).
Overall, we found 10 manuscripts that included A. americanum (Tables 1–3). The only distribution map for A. americanum in Illinois was by Springer et al. (2014). We base our distribution estimates on 4,045 records from primary data (90% of our 4,482 A. americanum records). We note the value of the primary data as these data sources allowed us to determine the establishment of a tick species in a county based on life stage, even when the numbers of ticks were less than six. We updated the status for A. americanum in 12 counties out of 29 counties newly defined as established, based only on life-stage information. The impact of primary data with clear and specific tick-level information including, the location of tick collection, life stage, and the number of ticks, is noteworthy.
Passive surveillance where tick specimens are voluntarily submitted to state or federal public health agencies is a tool to identify species presence (Childs and Paddock 2003, Springer et al. 2014, Barrett et al. 2015, Pak et al. 2019). However, it may not provide enough information to validate the origin of every tick collected. The effect is a tick report in a county when the tick came from a neighboring county or state for a tick collected from a highly mobile wildlife host such as a deer or bird.
The state or county of origin (where the host picked up the tick) may be different from the county of collection for ticks collected from a highly mobile wildlife host such as deer or birds. However, our tick occurrence record was the county of tick collection from the wildlife host. Similarly, a record of tick occurrence in a ‘new’ county from a human host without a travel history does not necessarily reflect on the life cycle and reproducing population of that tick in the county. While the lack of travel history of a human host or from a host associated with human movement (pet or livestock) is a limitation of this study, it points to the need of capturing this information to improve estimates of risk exposure and spatial certainty of the tick distribution, occurrence, and establishment by county.
Increased tick reports from passive surveillance may serve to recommend active surveillance (i.e., use of systematic field collection efforts to actively collect tick specimens using tick drags, flagging and removal of ticks from hosts in a county or location), to identify reproducing tick populations, and to determine the occurrence and status of a tick species in a county.
Overall, the literature search revealed a limited number of tick records that included both spatial and temporal information. Several reports in the literature did not provide exact numbers, but instead defined the status of I. scapularis and A. americanum as ‘established’, ‘reported’, or ‘not reported’ in a county (Dennis et al. 1998, Springer et al. 2014, James et al. 2015, Eisen et al. 2016), which was informative to meet the objectives of those manuscripts, but did not allow us to quantify their information and tally with the newly identified number of ticks from tick records in this work. This information may be more valuable when we try to determine density, abundance, and establishment of these ticks in a county.
We found that most secondary and tertiary sources (peer-reviewed literature) lacked specific tick-level information such as the number of ticks, life stage, and county of collection for a tick, collection-year, or day collected. Therefore, we note the value of reporting non-aggregated numbers of ticks, tick species, specific location, and exact date of collection within the publications. Many datasets lost in old technology storage units, disks, unreadable paper records are not retrievable, and we were not able to find the data from deceased colleagues, further supporting the value of publishing the dataset. This detailed and specific information can further our understanding of tick expansion, distribution, and risk of human exposure to tick bites.
Enlisting citizen scientists and supporting vector control staff in public health departments and even Illinois Mosquito Abatement Districts (some of which also collect tick data) can provide the means to collect entomological surveillance data. Their support can favor long-term research on the ecology, temporal distribution, and seasonal variation of tick populations that help researchers, scientists, and public health officials to identify areas of a substantial risk of exposure to tick bites. By supporting current and future tick identification, surveillance and tick research efforts, we can expand on the knowledge of spatial-temporal distribution and expansion of these vectors, inform risk assessments, direct and focus recommendations to citizens and their pets, and increase awareness among the medical community and the public at the regional and local level.
This study was not intended to evaluate variations in spatial and temporal trends in tick abundance, distribution or occurrence, or their relation to variation in tick-borne diseases in Illinois. However, we note the increase in tick-borne diseases from 1990 to 2017 that could explain an increase in the public interest, awareness, and first tick reports for A. americanum, and I. scapularis after 2000 (Fig. 1). Studying the relation between disease and tick trends on the geographical landscape could inform disease risk models and serve as a reminder of the medical importance of other tick species. Although I. scapularis is a tick species that warrants significant research and has been the most studied, other tick species cannot remain neglected.
We recognize the limitations of data collection in this study. We note the potential for duplicate records. One researcher may have published several manuscripts using the same dataset. Although we cross-referenced and evaluated publications by the same author if the tick information by year at the county level was the same across manuscripts for a researcher, we listed only one manuscript in the reference tables. Nonetheless, sometimes, it was unclear if tick records from manuscripts existed in public tick-data sources, entomological collections, or IDPH data, and consequently, we may have missed the identification of some duplicate records.
The sources of data differed, but so did the year and collection methods. New establishment of tick species can only be accurately assessed if data collection methods are comparable across time and geographical landscape. The heterogeneity of data and lack of harmonization of data collection across time and space makes it very difficult to evaluate the exact temporal changes and spatial trends in tick reports and establishments, or to make precise estimates about timelines for expansion and establishment of these species. To evaluate these changes, we would require the same or very similar field data collection methods implemented at the same location at different times. Thus, we emphasize the need for a national tick database with raw and specific tick-level data that allows for the comprehensive undertaking of the evaluation of temporal tick expansion over time and space.
This work was not intended to be a systematic review nor a survey design effort. Although we used tools to select, assess, include or exclude published manuscripts following the basic structure of a systematic review; and, although we reached out to past and present collaborators, we did not follow a survey design to capture all possible available data. As such, our results only reflect occurrence and status change based on our best available knowledge. We assume that we missed data sources. Therefore we cannot make final and definitive conclusions about temporal changes in tick expansion.
We identified over 18,000 individual tick records; this is likely an underestimate because not all studies incorporated into our dataset provided specific tick numbers. For example, if an author recorded 102 deer infested with a species of the tick but did not provide the number of ticks per deer, we estimated a minimum number of 100 ticks, represented as 100+ (Table 3). However, we did not include those estimates in the calculations of tick numbers to update the status in Fig. 2 or the text because the numbers may have been off by unknown orders of magnitude.
We chose the conservative approach of using precise tick numbers to inform our estimates of status updates by county. In Table 4, we illustrate the contributions of primary and secondary data sources, and the lost opportunity to gain specific tick numbers from tertiary data sources.
We integrated historical tick information for Illinois by including data from publications without the tick-level data (i.e., we lacked raw data). Often, ticks were collected by different groups, data were aggregated for the range of years of collection, reported without life stage, or there was a delay in tick identification or data sharing with IDPH at the time of this work. Our status and distribution maps and tick numbers (Supp Table S1 [online only]) highlight the lack of tick data for some counties in Illinois and serve us to focus on future surveillance efforts.
Conclusion
This manuscript updates the first reports, occurrence, distribution, and status of the three main vector tick species in Illinois: A. americanum, D. variabilis, and I. scapularis in 77%, 96%, and, 75% of the 102 counties. Ixodes scapularis has been the primary focus of tick studies in Illinois (Tables 2 and 3). The relation between I. scapularis density, and the risk of human exposure to a tick bite is unclear. However, we suggest that additional surveillance is warranted in the central and southern parts of Illinois (Fig. 2) (CDC 2019a), where the tick is either not reported, or reported but with a remaining potential for becoming established.
We were unable to estimate precise timelines for expansion and the establishment of these ticks in light of the heterogeneity of the data. However, we document temporal and increasing trends in the number of counties with tick first report, and with a status updated over recent decades. Our depiction of the data helps to visualize temporal differences in tick occurrence (Fig. 1) and to identify areas in need of additional surveillance (Fig. 2).
We often attributed the determination of the distribution and status of the three tick species to unexplored primary tick-level data. Primary data provided the most comprehensive tick-level information followed by secondary and tertiary data. Primary data allowed for the determination of status by the number of ticks and life stage, it provided the highest number of tick records, and it was easier to extract their information compared to the other data sources. Voluntary submissions from passive surveillance efforts and tick collections provided higher numbers of ticks from more counties compared to secondary and tertiary data sources (Table 4).
Our updated information indicates that all three tick species are widespread in Illinois and established in many counties. Our work may serve to recommend active surveillance to identify reproducing tick populations at predetermined locations and to determine the occurrence and status of a tick species in a county. We highlight the need for tick surveillance efforts in areas without tick records or with a reported status.
Some of the detailed tick information from secondary or tertiary was lost in aggregated or summary data. However, there may be lost records in unaccounted entomological collections with identified and unidentified tick specimens. Some of the specimens and data responsible for our updates existed for several years but remained unknown. It is unclear how many specimen collections we missed, yet their contribution to the understanding of the geographic and temporal expansion of these ticks may be significant. We found records that were lost because the tick collections were unknown, the specimens from entomological collections were not identified, archived, or digitized until recent years; or, the tick-level data were not published with the manuscripts.
Although not every tick carries a pathogen, our updated maps can inform the medical community about the occurrence of these disease vectors in the area. Increase awareness of tick occurrence may lead to the consideration of differential diagnosis that improves recognition and reporting of tick-borne diseases.
We note that changes in the visual trends of diseases transmitted by ticks suggest the importance for understudying the occurrence, status, and distribution of ticks of medical significance of humans and domestic animals. Both passive and active surveillance play a role in this effort. For example, the incidence of the emerging pathogen Ehrlichia chaffeensis is 10-fold higher based on active versus passive surveillance (Paddock and Childs 2003). However, passive surveillance can reveal if a tick species is present in an area. For example, Amblyomma maculatum (Koch) was detected in 2008–2009 in Johnson and Union Counties in southern Illinois (NE Mateus-Pinilla passive surveillance based on voluntary submissions). Amblyomma maculatum is a known vector of Rickettsia parkeri (Paddock et al. 2010), an emerging spotted fever pathogen. Our findings support the need for additional efforts to determine the distribution and occurrence of this tick in Illinois. After all, it is essential to determine the spatial, temporal, and habitat information related to the distribution of ticks (Nieto et al. 2018) and the tick-borne diseases that they transmit.
Cases of tick-borne diseases are increasing in Illinois. However, the extent of human exposure to these ticks requires further assessment (Herrmann et al. 2014). The status updates from this manuscript facilitate directing future studies, disease and tick surveillance efforts and assessments of risk of exposure in areas with and without the vector. Besides, it will contribute to the development of risk models for tick-pathogen transmission in Illinois.
There is a need for a systematic, national vector surveillance program that supports publishing tick-level data including collection method, tick species, number of ticks, life stage, specific date, and location of the collection. The program can encourage and support sharing data with public health agencies and conducting risk analyses of human exposure to tick bites and tick-borne diseases. A national vector surveillance program can intensify surveillance efforts, improve synergies between researchers and agencies conducting target outreach to the public, and supporting the detection of tick collections that may remain to be identified, digitized, and shared. The program can emphasize the importance of sharing raw data, using multiple surveillance methods, and submitting voucher specimens to entomological collections that keep accurate and organized data records shared with state public health units. We recognize the value of creating guidelines to facilitate the sharing of data by the scientific community where scientists receive some credit in support of the scientific and academic expectations of their institutions.
The data recovery effort is a powerful tool to evaluate the historical status and changes in distribution, expansion, and invasion of ticks, and it sets an opportunity to guide future studies. We assert the value in searching for unused or underused data sources, funding strategies to identify, archive, maintain, report tick data to state health agencies like IDPH, as well as to sites broadly accessible to researchers (e.g., the CDC’s tick module in National Arboviral Surveillance System ArboNET https://wwwn.cdc.gov/arbonet/). We emphasize the importance of conducting active surveillance in counties with tick reports and counties contiguous to areas with tick reports and with established tick populations.
Acknowledgments
We thank Drs. J.A. Nelson, U. Kitron, and J. Rydzewski for contributing data to this manuscript, Bennett Lamczyk and Alvyn Gonzalo Hernández Reyes for assistance with proofreading and reference management, Nelda A. Rivera for assistance with reviewing and editing the manuscript and Melissa Kehart for financial support to identify ticks. We also thank the Illinois Department of Public Health, TickReport, Kendall County Health Department, Forest Preserve District of DuPage County, United States National Tick Collection, Tommy McElrath (Illinois Natural History Survey - Insect Collection) for their contributions to tick records. We conducted this research through collaborative efforts among the Upper Midwestern Regional Center of Excellence for Vector-Borne Disease; the University of Illinois and the Illinois Natural History Survey - Prairie Research Institute. We thank the Illinois Natural History Survey - Prairie Research Institute for funding and facilities. This publication was supported by Cooperative Agreement #U01 CK000505, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers of Disease Control and Prevention or the Department of Health and Human Services.
Disclaimer: The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
References Cited
Adams
, D. A., K. R. Thomas, R. A. Jajosky, L. Foster, P. Sharp, D. H. Onweh, A. W. Schley, W. J. Anderson, P. M. Arguin, A. Artus, et al. . 2016. Summary of notifiable infectious diseases and conditions — United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 63: 1–152.
Adams
, D. A., K. R. Thomas, R. A. Jajosky, L. Foster, G. Baroi, P. Sharp, D. H. Onweh, A. W. Schley, and W. J. Anderson. 2017. Summary of notifiable infectious diseases and conditions — United States, 2015. MMWR. Morb. Mortal. Wkly. Rep. 64: 1–143.
Allan
, B. F., H. P. Dutra, L. S. Goessling, K. Barnett, J. M. Chase, R. J. Marquis, G. Pang, G. A. Storch, R. E. Thach, and J. L. Orrock. 2010. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proc. Natl. Acad. Sci. 107: 18523–18527.
Anderson
, J. F., and L. A. Magnarelli. 1984. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J. Biol. Med. 57: 627–641.
Barrett
, A. W., B. H. Noden, J. M. Gruntmeir, T. Holland, J. R. Mitcham, J. E. Martin, E. M. Johnson, and S. E. Little. 2015. County scale distribution of Amblyomma americanum (Ixodida: Ixodidae) in Oklahoma: addressing local deficits in tick maps based on passive reporting. J. Med. Entomol. 52: 269–273.
Bestudik
, J. 2006. Surveillance is information for action: Lyme disease, Illinois Infect. Dis. Rep. 3: 3–5.
Bouseman
, J. K., U. Kitron, C. E. Kirkpatrick, J. Siegel, and K. S. Todd. 1990. Status of Ixodes dammini (Acari: Ixodidae) in Illinois. J. Med. Entomol. 27: 556–560.
Brownstein
, J. S., T. R. Holford, and D. Fish. 2003. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ. Health Perspect. 111: 1152–1157.
[CDC] Centers for Disease Control and Prevention
. 2018. Notifiable infectious diseases and conditions data tables. Available from https://wwwn.cdc.gov/nndss/infectious-tables.html
[CDC] Centers for Disease Control and Prevention
. 2019a. Data surveillance. Available from https://www.cdc.gov/lyme/datasurveillance/index.html
[CDC] Centers for Disease Control and Prevention
. 2019b. Geographic distribution of ticks that bite humans. Available from https://www.cdc.gov/ticks/geographic_distribution.html
[CDC] Centers for Disease Control and Prevention
. 2019c. Tickborne diseases of the United States. Available from https://www.cdc.gov/ticks/tickbornediseases/index.html
Chapman
, E. S., and T. E. Siegle. 2000. A novel method for tick retrieval reveals northern migration of Amblyomma americanum into Pike County, IL, USA. Int. J. Acarol. 26: 391–393.
Childs
, J. E., and C. D. Paddock. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Ann Rev Entomol. 48: 307–337.
Cortinas
, M. R., and U. Kitron. 2006. County-level surveillance of white-tailed deer infestation by Ixodes scapularis and Dermacentor albopictus (Acari: Ixodidae) along the Illinois River. J. Med. Entomol. 43: 810–819.
Cortinas
, R., and S. M. Spomer. 2014. Occurrence and county-level distribution of ticks (Acari: Ixodidae) in Nebraska using passive surveillance. J. Med. Entomol. 51: 352–359.
Cortinas
, M. R., M. A. Guerra, C. J. Jones, and U. Kitron. 2002. Detection, characterization, and prediction of tick-borne disease foci. Int. J. Med. Microbiol. 291: 11–20.
Dantas-Torres
, F., B. B. Chomel, and D. Otranto. 2012. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 28: 437–446.
De la Fuente
, J., A. Estrada-Peña, J. M. Venzal, K. M. Kocan, and D. E. Sonenshine. 2008. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13: 6938–6946.
Dennis
, D. T., T. S. Nekomoto, J. C. Victor, W. S. Paul, and J. Piesman. 1998. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 35: 629–638.
Eisen
, R. J., L. Eisen, and C. B. Beard. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 53: 349–386.
Estrada-Peña
, A., and J. De La Fuente. 2014. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 108: 104–128.
Fischer
, J., and C. Hilger. 2017. Alpha-gal syndrome: clinical presentation, new concepts, and unmet needs. Curr. Treat. Options Allergy. 4: 303–311.
French
, J. B., W. L. Schell, J. J. Kazmierczak, and J. P. Davis. 1992. Changes in population density and distribution of Ixodes dammini (Acari: Ixodidae) in Wisconsin during the 1980s. J. Med. Entomol. 29: 723–728.
Gill
, R. M. A., and V. Beardall. 2001. The impact of deer on woodlands: the effects of browsing and seed dispersal on vegetation structure and composition. Forestry. 74: 209–218.
Gilliam
, M. E., W. T. Rechkemmer, K. W. McCravy, and S. E. Jenkins. 2018. The influence of prescribed fire, habitat, and weather on Amblyomma americanum (Ixodida: Ixodidae) in West-Central Illinois, USA. Insects. 9: 437–446.
Goddard
, J., and A. S. Varela-Stokes. 2009. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 160: 1–12.
Guerra
, M., E. Walker, C. Jones, S. Paskewitz, M. Roberto Cortinas, L. B. Ashley Stancil, M. Bobo, and U. Kitron. 2002. Predicting the risk of Lyme disease: Habitat suitability for Ixodes scapularis in the north central United States. Emerg. Infect. Dis. 8: 289.
Hahn
, M. B., C. S. Jarnevich, A. J. Monaghan, and R. J. Eisen. 2016. Modeling the Geographic Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Contiguous United States. J. Med. Entomol. 53: 1176–1191.
Hamer
, S. A., T. L. Goldberg, U. D. Kitron, J. D. Brawn, T. K. Anderson, S. R. Loss, E. D. Walker, and G. L. Hamer. 2012. Wild birds and urban ecology of ticks and tick-borne pathogens, Chicago, Illinois, USA, 2005–2010. Emerg. Infect. Dis. 18: 1589–1595.
Herrmann
, J. A., N. M. Dahm, M. O. Ruiz, and W. M. Brown. 2014. Temporal and spatial distribution of tick-borne disease cases among humans and canines in Illinois (2000–2009). Environ. Health Insights. 8: 15–27.
[IDPH] Illinois Department of Public Health
. 2018a. Reportable communicable disease cases, 1990 – 1999. Available from http://www.idph.state.il.us/health/infect/communicabledisease 9099.html
[IDPH] Illinois Department of Public Health
. 2018b. Tickborne heartland virus case in Illinois. Available from http://www.dph.illinois.gov/news/tickborne-heartland-virus-case-illinois
[INHS] Illinois Natural History Survey Prairie Research Institute
. 2018. Illinois natural history survey, insect collection. Available from http://inhsinsectcollection.speciesfile.org/InsectCollection.aspx
James
, A. M., C. Burdett, M. J. McCool, A. Fox, and P. Riggs. 2015. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the U.S.A. Med. Vet. Entomol. 29: 178–188.
Jobe
, D. A., S. D. Lovrich, J. A. Nelson, T. C. Velat, C. Anchor, T. Koeune, and S. A. Martin. 2006. Borrelia burgdorferi in Ixodes scapularis ticks, Chicago area. Emerg. Infect. Dis. 2: 1039–1041.
Jobe
, D. A., J. A. Nelson, M. D. Adam, and S. A. Martin. 2007. Lyme disease in urban areas, Chicago. Emerg. Infect. Dis. 13: 1799–1800.
Jones
, C. J., and U. D. Kitron. 2000. Populations of Ixodes scapularis (Acari: Ixodidae) are modulated by drought at a lyme disease focus in Illinois. J. Med. Entomol. 37: 408–415.
Kitron
, U., J. K. Bouseman, and C. J. Jones. 1991. Use of the ARC/INFO GIS to study the distribution of Lyme disease ticks in an Illinois county. Prev. Vet. Med. 11: 243–248.
Kitron
, U., C. J. Jones, J. K. Bouseman, J. A. Nelson, and D. L. Baumgartner. 1992. Spatial analysis of the distribution of Ixodes dammini (Acari: Ixodidae) on white-tailed deer in Ogle County, Illinois. J. Med. Entomol. 29: 259–266.
Lantos
, P. M., J. Tsao, L. E. Nigrovic, P. G. Auwaerter, V. G. Fowler, F. Ruffin, E. Foster, and G. Hickling. 2017. Geographic expansion of Lyme disease in Michigan, 2000–2014. Open Forum Infect. Dis. 4: 1–5.
Lepitzki
, D. A., A. Woolf, and B. M. Bunn. 1992. Parasites of cottontail rabbits of southern Illinois. J. Parasitol. 78: 1080–1083.
Mannelli
, A., U. Kitron, C. J. Jones, and T. L. Slajchert. 1993. Ixodes dammini (Acari: Ixodidae) infestation on medium-sized mammals and blue jays in northwestern Illinois. J. Med. Entomol. 30: 950–952.
Minigan
, J. N., H. A. Hager, A. S. Peregrine, and J. A. Newman. 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Tick. Borne. Dis. 9: 354–362.
Mitcham
, J. R., A. W. Barrett, J. M. Gruntmeir, T. Holland, J. E. Martin, E. M. Johnson, S. E. Little, and B. H. Noden. 2017. Active surveillance to update county scale distribution of four tick species of medical and veterinary importance in Oklahoma. J. Vector Ecol. 42: 60–73.
Mixson
, T. R., S. R. Campbell, J. S. Gill, H. S. Ginsberg, M. V. Reichard, T. L. Schulze, and G. A. Dasch. 2006. Prevalence of Ehrlichia, Borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine States. J. Med. Entomol. 43: 1261–1268.
Montgomery
, G.G., and R.E. Hawkins. 1967. Lone star ticks from white-tailed deer in Illinois. Trans Ill State Acad. Sci. 60: 203.
Monzón
, J. D., E. G. Atkinson, B. M. Henn, and J. L. Benach. 2016. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol. Evol. 8: 1351–1360.
Nelson
, T. A., K. Y. Grubb, and A. Woolf. 1984. Ticks on white-tailed deer fawns from southern Illinois. J. Wildl. Dis. 20: 300–302.
Nelson
, J. A., J. K. Bouseman, U. Kitron, S. M. Callister, B. Harrison, M. J. Bankowski, M. E. Peeples, B. J. Newton, and J. F. Anderson. 1991. Isolation and characterization of Borrelia burgdorferi from Illinois Ixodes dammini. J. Clin. Microbiol. 29: 1732–1734.
Nieto
, N. C., W. Tanner Porter, J. C. Wachara, T. J. Lowrey, L. Martin, P. J. Motyka, and D. J. Salkeld. 2018. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS One 13: e0199644.
Oliver
, J. D., S. W. Bennett, L. Beati, and L. C. Bartholomay. 2017. Range Expansion and Increasing Borrelia burgdorferi infection of the tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J. Med. Entomol. 54: 1727–1734
Paddock
, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16: 37–64.
Paddock
, C. D., P. E. Fournier, J. W. Sumner, J. Goddard, Y. Elshenawy, M. G. Metcalfe, A. D. Loftis, and A. Varela-Stokes. 2010. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 76: 2689–2696.
Pak
, D., S. B. Jacobs, and J. M. Sakamoto. 2019. A 117-year retrospective analysis of Pennsylvania tick community dynamics. Parasites and Vectors 12: 189.
Rydzewski
, J., N. Mateus-Pinilla, R. E. Warner, S. Hamer, and H.-Y. Weng. 2011. Ixodes scapularis and Borrelia burgdorferi among diverse habitats within a Natural Area in East-Central Illinois. Vector Borne Zoonotic Dis. 11: 1351–1358.
Rydzewski
, J., N. Mateus-Pinilla, R. E. Warner, J. A. Nelson, and T. C. Velat. 2012. Ixodes scapularis (Acari: Ixodidae) distribution surveys in the Chicago Metropolitan Region. J. Med. Entomol. 49: 955–959.
Schneider
, S. C., C. M. Parker, J. R. Miller, L. Page Fredericks, and B. F. Allan. 2015. Assessing the contribution of songbirds to the movement of ticks and Borrelia burgdorferi in the Midwestern United States during fall migration. Ecohealth. 12: 164–173.
Schulze
, T. L., R. A. Jordan, and R. W. Hung. 2009. Effects of microscale habitat physiognomy on the focal distribution of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. Environ. Entomol. 31: 1085–1090.
Siegel
, J. P., U. Kitron, and J. K. Bouseman. 1991. Spatial and temporal distribution of Ixodes dammini (Acari: Ixodidae) in a northwestern Illinois state park. J. Med. Entomol. 28: 101–104.
Slajchert
, T., U. D. Kitron, C. J. Jones, and A. Mannelli. 1997. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern Illinois, USA. J. Wildl. Dis. 33: 40–46.
Sotala
, D. J., and C. M. Kirkpatrick. 1973. Foods of white-tailed deer, Odocoileus virginianus, in Martin County, Indiana. Am. Midl. Nat. 89: 281–286.
Springer
, Y. P., L. Eisen, L. Beati, A. M. James, and R. J. Eisen. 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med. Entomol. 51: 342–351.
Stannard
, L. J., and L. R. Pietscht. 1958. Ectoparasites of the cottontail rabbit in Lee County, Northern Illinois, Biol. Notes. 18: 1–18.
TickReport
. 2019. Available from https://www.tickreport.com
Tokarz
, R., S. Sameroff, M. S. Leon, K. Jain, and W. I. Lipkin. 2014. Genome characterization of Long Island tick rhabdovirus, a new virus identified in Amblyomma americanum ticks. Virol. J. 11: 26.
[USNTC] United States National Tick Collection
. 2019. Available from https://cosm.georgiasouthern.edu/usntc/
Yoder
, J. A., and J. B. Benoit. 2003. Water vapor absorption by nymphal lone star tick, Amblyomma americanum (Acari: Ixodidae), and its ecological significance. Int. J. Acarol. 29: 259–264.
Zieman
, E. A., F. A. Jiménez, and C. K. Nielsen. 2017. Concurrent examination of bobcats and ticks reveals high prevalence of Cytauxzoon felis in Southern Illinois. J. Parasitol. 103: 343–348.
Author notes
These first authors contributed equally to this article.
These authors were co-principal investigators.
© The Author(s) 2019. Published by Oxford University Press on behalf of Entomological Society of America.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com
Supplementary data
tjz235_suppl_Supplementary_Figure_S1 - png file
tjz235_suppl_Supplementary_Table_S1 - docx file